What's up with Ferulic acid?
Last week we discussed Ferulic acid (FA), and one of you asked an excellent question regarding the ultimate fate of antioxidants like Ferulic acid. We've mentioned that upon scavenging a free radical, Ferulic acid forms a stable radical that doesn't attack cells and DNA. So what happens then? Does it just boringly saunter around your skin, judging you for your lack of sunscreen? Well, as it turns out, FA's ultimate demise is more interesting than how Daenerys was killed in Game of Thrones.
Recap on free radicals and antioxidants
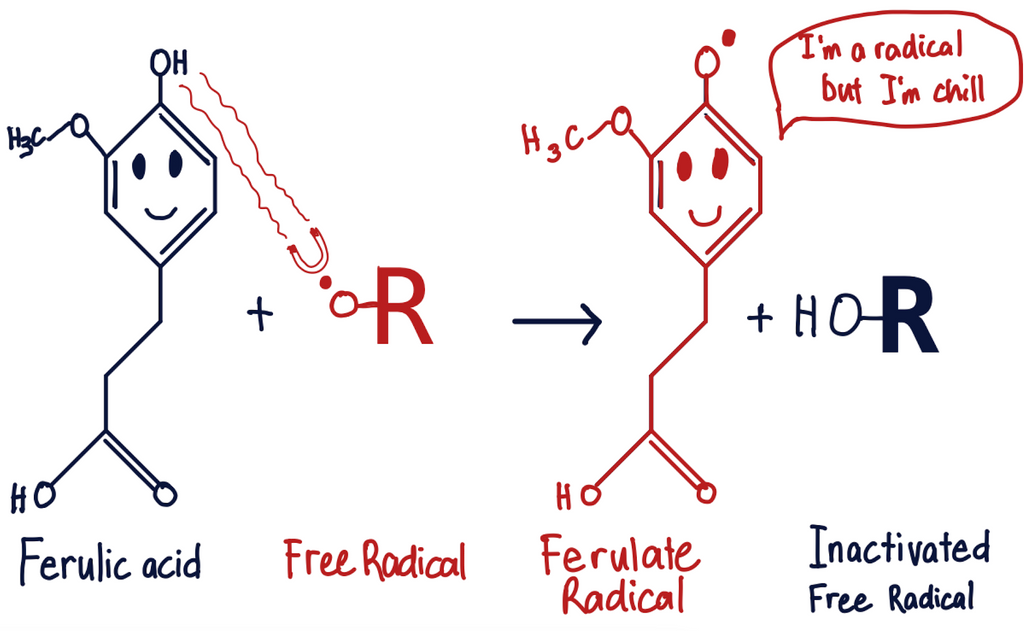
Prepare to be amazed
As it turns out, the Ferulate radical's ultimate fate can be one of three scenarios:

- It can take out another radical through radical-radical coupling to form various non-radical ferulates. The major product is 5-hydroxyferulic acid which can AGAIN neutralize TWO MORE FREE RADICALS!
- It can donate another H atom to another radical and cyclicize
- Finally, it can dimerize with itself (bind with another ferulate radical) to produce a brand new, fully-functioning antioxidant (Shoooookkeeeth).
Dimerization is the most energetically feasible reaction among the three mechanisms. In fact, ferulate radicals readily decay into a C5-C5 dimer with more anti-radical properties than the original ferulic acid [^1]. Aside from the major product, dimerization of the ferulate radical can also form other antioxidants like Curcumin or FA-2, which has a more potent anti-inflammatory ability than Ferulic acid [^2]. In a nutshell, two dying Ferulic acids found love, became one and regenerated into a better antioxidant.
But that's not all. Ferulate radicals can also bind with other antioxidant radicals to form antioxidants capable of terminating radical chains (most of which are still uncharacterized). The possibilities are endless!
One door closes, another one opens
How exciting is that? Ferulic acid oxidization leads to so many possible outcomes and many more ways to scavenge free radicals. Because of these mechanisms, FA is considered a great secondary antioxidant to boost the effectiveness of other antioxidants. It supports and stabilizes other antioxidant radicals and in a way renew them. This allows the antioxidant system to quench double, or even triple numbers of free radicals than what they can do individually.
If it's so good, why don't we smother our faces with Ferulic acid 24/7?
Well, FA is a good antioxidant but not as great as others like Catechins (EGCG). Besides, like Ascorbic acid, it can act as a pro-oxidant and produce free radical species in the presence of transition metals like Iron and Copper ions [^3]. For these reasons, it is super duper important to have various antioxidants and metal chelators in an antioxidant formulation like in our Vitamin X. Wink Wink.
Until Next time.
References
[^1]: Amić, A., Marković, Z., Dimitrić Marković, J., Milenković, D., & Stepanić, V. (2020). Antioxidative potential of ferulic acid phenoxyl radical. Phytochemistry, 170, 112218. doi: 10.1016/j.phytochem.2019.112218
[^2]: Ou, L., Kong, L., Zhang, X., & Niwa, M. (2003). Oxidation of Ferulic Acid by Momordica charantia Peroxidase and Related Anti-inflammation Activity Changes. Biological And Pharmaceutical Bulletin, 26(11), 1511-1516. doi: 10.1248/bpb.26.1511
[^3]: Truong, D., Nhung, N., & Dao, D. (2020). Iron ions chelation-based antioxidant potential vs. pro-oxidant risk of ferulic acid: A DFT study in aqueous phase. Computational And Theoretical Chemistry, 1185, 112905. doi: 10.1016/j.comptc.2020.112905
