Does Hyaluronic acid dehydrate the skin?
In December, we tested individual humectants on their ability to hydrate the skin. One group we tested were polymeric humectants, which included various Molecular Weights of Hyaluronic acid. The results of the study were a bit shocking. To sum it all up, most of the Hyaluronic Acid (HA) samples dehydrated the skin. We had to remeasure everything and make sure our controls were working properly, but the results stayed the same for all Hyaluronic acid humectants. We also sourced HA from different suppliers, and they all have the same results.

We've been going back and forth analyzing these results. It is most likely that Hyaluronic acid is highly dependent on the relative humidity of the environment, meaning, in low relative humidity (RH), it can dehydrate the skin.
But how is this possible?
One unique thing about HA that differs from most humectants is its ability to affect keratin secondary structures and barrier lipid organization. One study found that 5 kDa HA changed the structures of keratin from alpha-helix to B-sheet, resulting in a more disorganized arrangement. This interconversion allows low MW HA to hydrate the skin quickly and at the same time allow penetration of substances. Interestingly, 100kDa and 1M kDa didn't show significant changes in keratin structure. 1
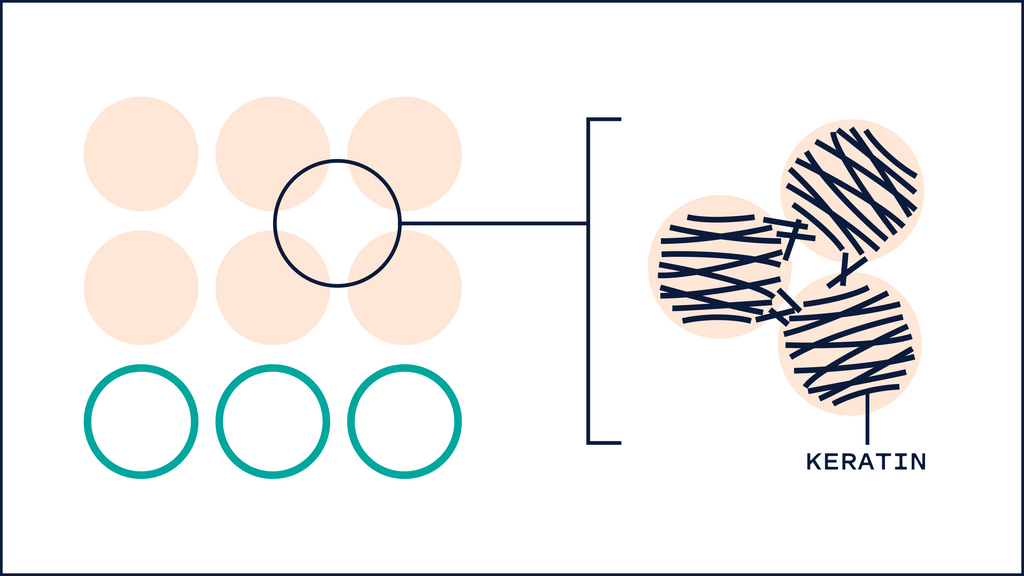
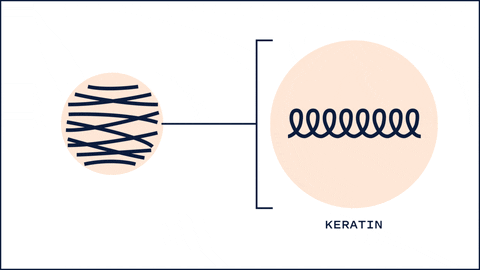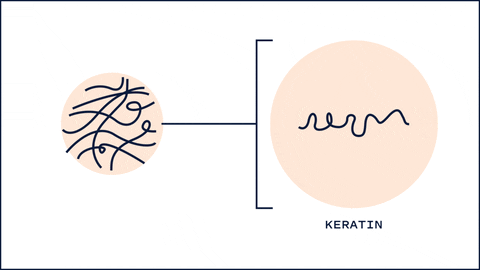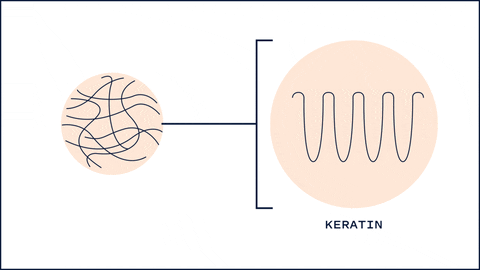
100 kDa HA, on the other hand, had more of an effect on the barrier lipids. Typically, these lipids (Ceramides, Cholesterol and Fatty acids) are packed tightly to prevent water loss and entry of substances. 100 kDa HA disrupted this organization, allowing more water to flow into the upper layers of the skin. On the other hand 1M HA showed no disturbance in the barrier lipid organization.
Other humectants typically do not affect the organization of the barrier lipids. Small polar molecules like Glycerin and Urea usually lodge themselves between the layer of polar heads of the barrier lipids so they don't disrupt their organization, evidenced by an absence of change in the FTIR spectra of barrier lipids. They also don't affect the secondary structure (motifs within a protein) of keratin, but they do affect its tertiary structure (the global folding of the protein) to hold more water. This results in increased water holding capacity of the Stratum Corneum without affecting the integrity of the barrier lipids.
Under normal Relative Humidity (50%), low MW HA opens up the barrier to hydrate the uppermost layer of the skin. Since evaporation on the skin's surface is normal, the SC gets sufficiently hydrated without losing a lot of it to evaporation. When the RH drops (air is dryer), there is significant evaporation on the skin surface. Since the barrier is open, most water from the dermis gets pulled towards the upper layers, evaporating quickly. You end up with a dehydrated Stratum Corneum unable to keep up with surface evaporation.
However, this barrier atlering ability was only seen in the lower molecular weight HA and not in high molecular weight of 1M Daltons. Intriguingly, even the higher molecular weight HA dehydrated the skin. Another interesting fact is that 3 Million Da HA was more dehydrating than 1 M HA. This result downplays the barrier altering effect as the cause of dehydration because high molecular weight HA does not alter the barrier and keratin structure.
Important Caveat
The tests that we've done are on short-term hydration. Hyaluronic acid has been studied to have a more long-term effect by upregulating genes associated with hydration. (Side note: HA needs to penetrate the skin for these genes to be upregulated. However, only low MW HA can penetrate the skin, but we're not sure what the whole picture looks like in terms of its effect. Some are cautioning about low MW HA being pro-inflammatory, but that is for a much, much longer discussion.)
Purpose
Okay, so let's say we are betting on HA's longer-term hydration effect. Our previous humectant combination study resulted in a combination that works well in hydrating the skin. We wanted to know how it plays well with other humectants, so we studied how HA would affect skin hydration when added to this humectant combination. Our thought process is that if HA at minimum doesn't decrease hydration of the humectant combination, then we can add it for longer-term hydration.
Procedure:
- n=8
- measurement with Corneometer CM825
- au values minus baseline = change in hydration
- t=30 minutes after application
- RH=20%
Solutions Tested

Results


Well, that sucks. We were hoping to add HA because it adds a characteristic slip-and-glide to the formulation, plus we were betting on its longer-term hydration. However, as you see here, it significantly decreased the hydration of the humectant combination. The results also confirmed the previous results; the decrease is more apparent in 3M Da HA than 1M HA. When combined, it seems that 3M further decreased the hydration ability of 1M Da HA.
Discussion
Theoretically, HA of these sizes shouldn't affect the barrier at all. They can't penetrate the skin, so they won't have a pronounced effect on the barrier. At first, we thought it could also be that HA forms a film and the Corneometer only measures a specific depth (10-20 um). However, the films formed with 1% HA are only at about 1um, and HA-Crosspolymer (which has a much higher molecular weight) didn't result in decreased hydration. This rules out the depth factor.
We've always dismissed the idea that HA serums shouldn't be used in low humidity as they may pull water from your skin rather than from the environment. Our argument was that a humectant cannot discern where to pull water from, it just pulls water wherever water is easily obtained. To add to that, any humectant for that matter will likely lead to a decreased hydration in lower relative humidity. However, given these results, we have to rethink that as they clearly show that HA does dehydrate the skin in lower humidity.
We still don't think that HA mainly pulls water from the environment. Because of our body temperature, evaporation is inevitable on the skin's surface. What is more likely happening is that in lower humidity, evaporation is higher on the skin surface compared to higer humidity. However, this doesn't answer why high MW HA dehydrates the skin without affecting the barrier organization and keratin structure
We're quite perplexed with how it dehydrates the skin but the results do show that it does dehydrate the skin at low humidity. Of course we need more samples to this study but it is a proof-of-concept for further studies down the road (Once we have more money lol). These results left us with more questions than answers: At which RH does it turn beneficial? How does HA perform in longer tem? If it doesn't affect the barrier nor the keratin structures, then how does it dehydrate the skin? Is it possible that there is a confounding factor to the study?Further tests are needed to figure these out. What's certain is that we won't be adding HA (except for the HA Crosspolymer that is already in the humectant combination) in the humectant combination. We're planning to acquire a Raman Spectroscopy later, which might help us understand what is happening layer by layer in the skin. If you have any suggestions or ideas, shoot us an email.
Reference
1: Witting, M., Boreham, A., Brodwolf, R., Vávrová, K., Alexiev, U., Friess, W. and Hedtrich, S., 2015. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Molecular Pharmaceutics, 12(5), pp.1391-1401.
